Harmony COVID-19: A ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection | Science Advances

Isothermal SARS-CoV-2 Diagnostics: Tools for Enabling Distributed Pandemic Testing as a Means of Supporting Safe Reopenings | ACS Synthetic Biology
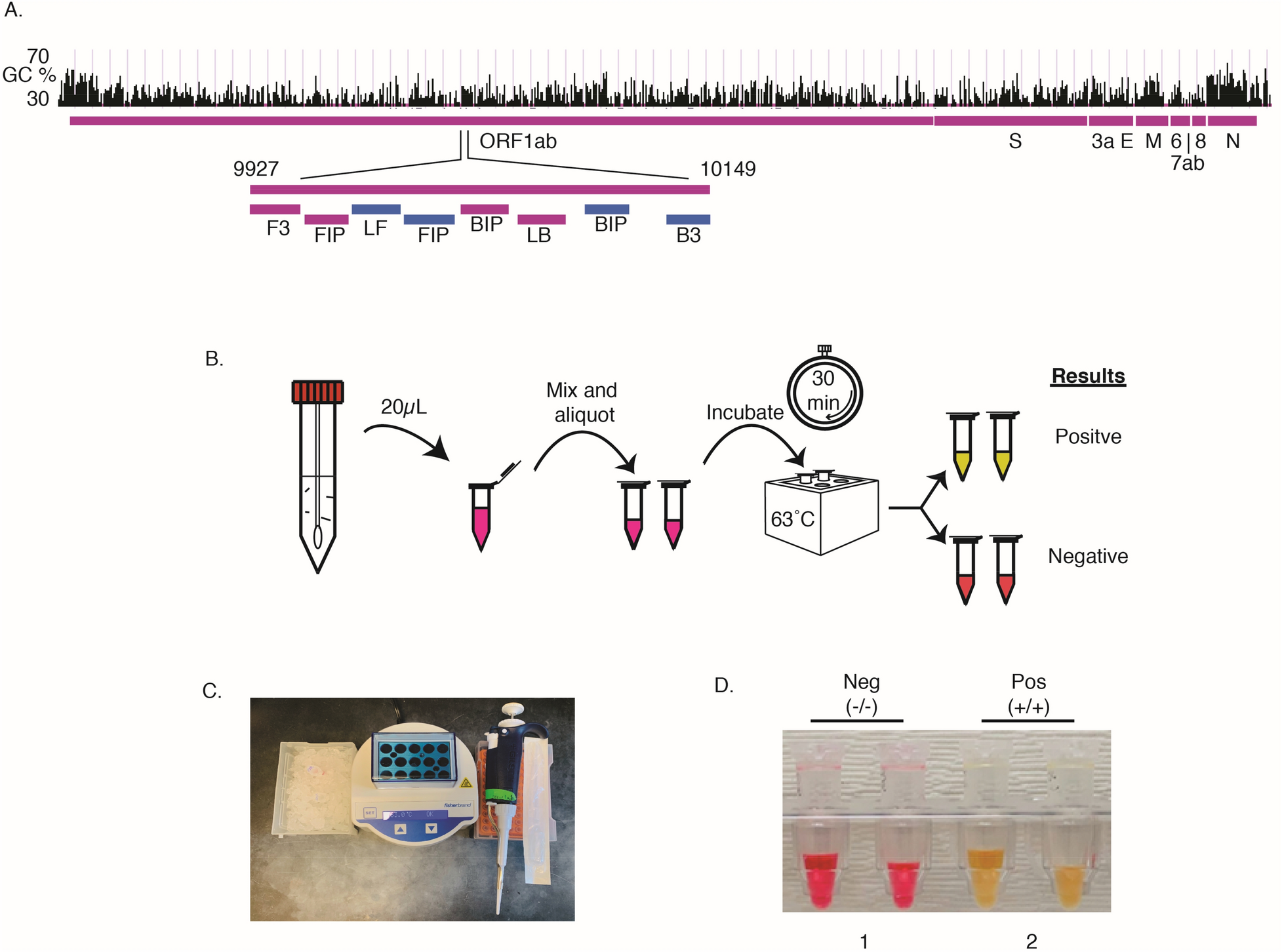
Direct diagnostic testing of SARS-CoV-2 without the need for prior RNA extraction | Scientific Reports

Jam-packed, 55-and-older event day before Gov. Abbott COVID-19 diagnosis had no masks, little distancing | KXAN Austin

Clinical validation of colorimetric RT-LAMP, a fast, highly sensitive and specific COVID-19 molecular diagnostic tool that is robust to detect SARS-CoV-2 variants of concern | medRxiv

Diagnostic performance of a colorimetric RT -LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa - eClinicalMedicine

Virus-Like Particles as Positive Controls for COVID-19 RT-LAMP Diagnostic Assays | Biomacromolecules

Rapid SARS-CoV-2 testing: LAMP and antigen COVID-19 tests promise inexpensive, accurate, and fast results to help manage the pandemic | Clinical OMICs

Abbott on Twitter: "“By the end of this year, we will have shipped globally over 300 million COVID tests across all of our different platforms.” – Robert Ford, our President and CEO,
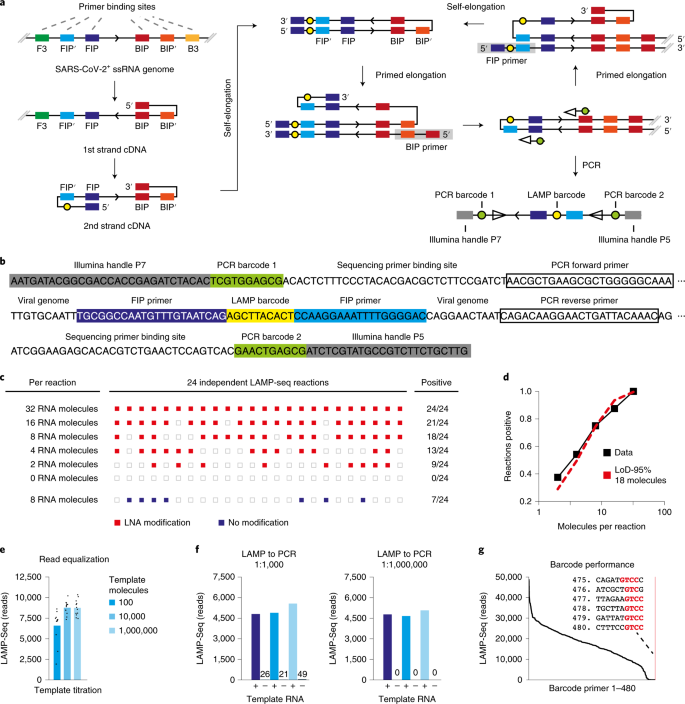
LAMP-Seq enables sensitive, multiplexed COVID-19 diagnostics using molecular barcoding | Nature Biotechnology



.jpg)







/cloudfront-us-east-1.images.arcpublishing.com/gray/N7EEZNRG2BCZDF576YPIPJU3LY.jpg)