Abbott issues firmware update for 350,000 defibrillators with cybersecurity vulnerabilities | Fierce Healthcare
New £1.8m clinical trial to help reduce sudden cardiac deaths using implantable defibrillators | Southampton Clinical Trials Unit | University of Southampton

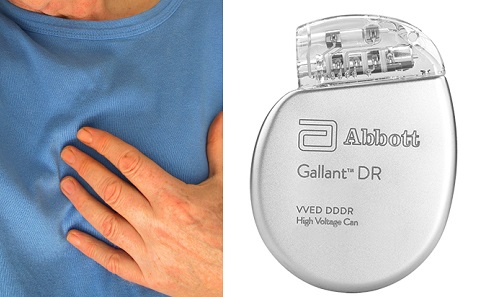
FDA Clears Abbott Gallant ICD and CRT With Bluetooth Connectivity and Continuous Remote Monitoring | DAIC